Check out this awesome infographic describing the chemical nature of baking.
Mmmmm… cake….
I have heard a number of times the similarities between cooking/ baking and science in a lab. Throw together a few ingredients, stir them, heat them (I’ve even used a scientific form of ‘microwave’), and voila! Delicious products. Although you probably shouldn’t eat the laboratory ones. Unless you really, really, REALLY want superpowers and aren’t afraid to die in your attempt.
Don’t we all…
The similarities don’t end there. Heat it for too short a period, chances are you’ll end up with a gooey mess (assuming that’s not what you wanted). Heat it for too long or set the temperature too high, blackened mess. Use out of date or impure starting materials, forget to add something, and you also miss out on synthesising your desired product.
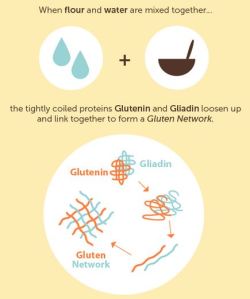
This infographic (originally posted on Shari’s Berries) shows just that, and it brings together many of the elements of the food science unit I teach to first years: Proteins (in eggs, milk, and flour), fats (in milk, butter and oils), and carbohydrates (in flour and sugars), as well as yeast, baking soda, and water. All of these ingredients come together to perform separate roles. For example, one role of the fats and oils in baking is to repel water (have you ever noticed how oil and water don’t mix? Here’s why: Polar and Non-Polar Compounds – ignore the ‘practical’ explanation). So by repelling water, water is no longer able to interact with certain proteins, in particular gliadin and glutenin, which together with water make gluten (I’ll be posting soon about gluten). If lots of gluten forms quickly, you end up with a dough that doesn’t rise well. So by using fats to repel water, there is a slow and steady production of gluten, which leads to a nicely formed dough which will rise well.
Side note: Gluten is not the enemy (unless you have coeliac disease).
Another example of the chemical interaction of a baking ingredient is baking soda. Baking soda is known as sodium bicarbonate, and is a ‘leavening agent’. This means that when it reacts with acids in the baking mixture, it releases carbon dioxide. It is this carbon dioxide that helps to form bubbles in the dough, helping it to rise.
It’s okay, carbon dioxide in your bread won’t kill you.
So the next time you’re planning on baking a cake, think about why you add so many different ingredients for a perfect, delicious product.
And then bring me some cake. I love cake.




Cooking’s just chemistry with style.
And taste!